Many people are looking for more effective natural ways to assist with what ails them. This coincides with CBD and other cannabis-related products hitting the market as a mainstream alternative, which is somewhat of a perfect storm.
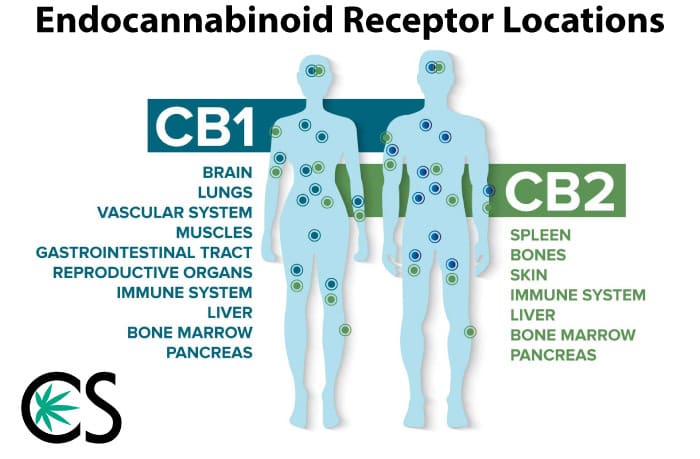
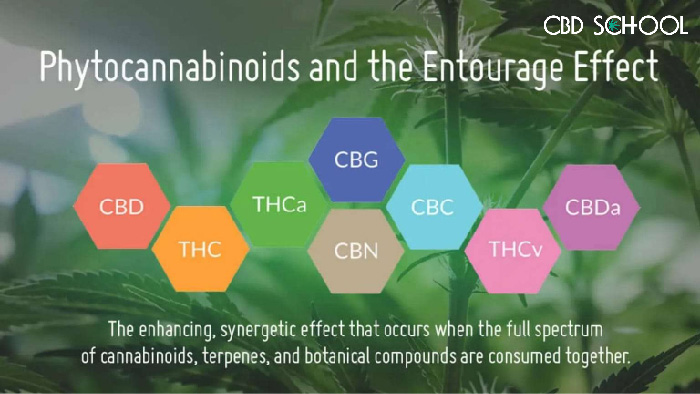
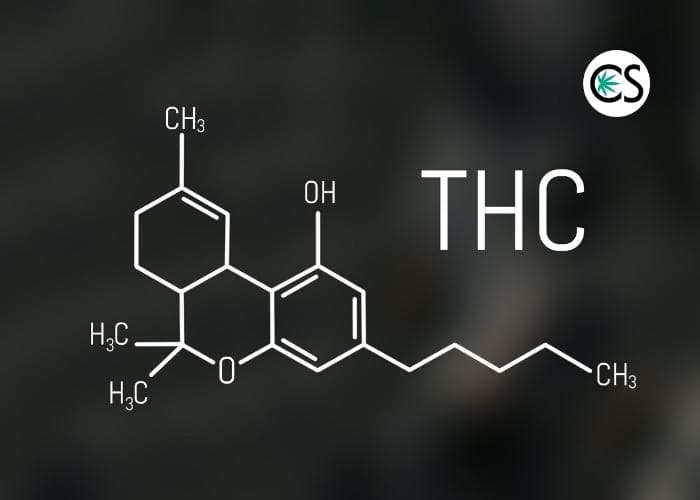
I’m sure CBD, at least the term, isn’t new to your vernacular, but as a quick refresher, CBD is a cannabinoid that is found in the cannabis plant, along with about 100 other cannabinoids, including THC, THCa, CBG, CBC, and Delta-8 THC.
We receive many questions at CBD School, and one of the most frequent is, “how much CBD can I take?
Before we dive in to answer that question, let’s tackle what people are turning to CBD for to assist with their ailments.
What Is CBD Used For?
While there have been many studies performed that look at the efficacy of CBD, and many people have heard about some of the illnesses that CBD has shown possible positive effects on assist with, like chronic pain1, arthritis2, insomnia3, and anxiety4, many more studies need to occur prior to CBD stepping out of the grey area that it currently sits.